Articles
Volumetric Karl Fischer Titration
posted : 17 دی 1399
By : Kianshar D Research Team
Karl Fischer titration is a specific technique for determination of the water content in different samples and is commonly used in the analysis of pharmaceuticals , petroleum , chemicals , resins , adhesives and many other products.
Karl Fischer titration is a method which is originally based on the famous Bunsen reaction:
SO2 + I2 + 2H2O -------> H2SO4 + 2HI
The Bunsen equation was revised several times during decades and finally changed to the below one which is known as the Karl Fischer equation :
H2O+ I2 + [RNH]+SO3CH3- + 2 RN → [RNH]+SO4CH3- + 2 [RNH]+I-
According to equation the KF reagent containing Iodine is consumed until all the water content of sample is used up. The excess iodine is appeared at the end point which is detected by the indicator. The water is consumed during the oxidation of the alkyl sulfite intermediate by iodine. The Karl Fischer reaction is pH sensitive and needs a base in order to proceed.
Two Karl Fischer titration methods
There are two Karl Fischer titration methods : volumetric KF Titration and coulometric KF Titration. In the volumetric methodwhich is our topic in this article , the KF reagent containing iodine is added to the solvent , usually methanol in the titration cell. In this titration the end-point is achieved when the iodine stops reaction with the water content of the sample and the water content is calculated from the volume of the used Karl Fischer reagent .
Figure 1 : Comparison between volumetric and coulometric Karl Fischer titration methods
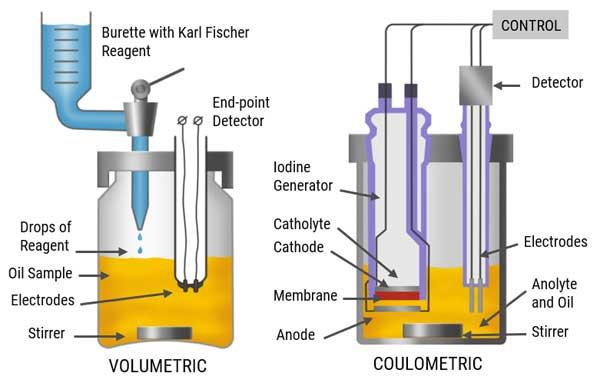
It is possible to determine the water content from 0.01% to 100% by volumetric KF titration method. This is a wide range which makes the volumetric KF titration as the most commonly used KF titration method.
Karl Fischer Titration reagents
Volumetric Karl Fischer titration is performed by two kinds of reagents ;
one component or two component. One component reagent practically is a solution containing all the necessary reagents for KF titration.
In two-component reagents, the needed ingredients for the Karl Fischer reaction are provided in two separate reagents, titrant and solvent. The titrant consists of a solution of iodine in methanol while the solvent that is used as the working medium in the KF cell contains the other Karl Fischer reaction components - sulfur dioxide and a suitable base dissolved in methanol.
Two-component reagent systems have a greater long-term stability and a slightly faster titration speed than one-component reagents. But using the one component reagents are more simple and this makes them prevailing KF reagents.
It is important to understand that the water equivalents* of the KF reagents change over time and every experienced user knows that KF reagents must be calibrated regularly in order to revise the water equivalent in the calculation formula of determination of the water content. The calibration is easily done using certified water standards.
Some applications of volumetric Karl Fischer titration
Determination of water content is a very important analysis which is in interest of many quality control and research labs which are engaged with pharmaceutical drug formulation , estimation of shelf life of products , evaluation of products quality and ...
Here we can name some of the applications of volumetric KF titration as below :
- Chemicals
- engine coolant
- Pharmaceuticals
- Petroleum and plastics
- Power stations
- Foodstuffs
- oil seeds
- Adhesives and paints
Advantages of volumetric Karl Fischer titration
Among many advantages of the volumetric Karl Fischer titration by automatic KF titrators we can poin to the below instances:
1- determination of water content in most cases is done by volumetric KF titration within a few minutes.
2- It is the best preferred method for determination of H2O among all the volatile substances in a sample.
3- The volumetric KF titration has excellent accuracy.
4- The method is able to determine the water content over a wide range between 0.01% to 100%.
KF Titration : Easy by a modern automatic KF Titrator
Karl Fischer is a kind of titration which uses some toxic reagents and samples. This warns everybody to be careful during the process and avoid each possible health danger. Fortunately the KF titration has been fully automated since years ago and there is no any risk to do KF titrations in labs. The modern Karl Fischer titrators such as KFT50 Titrika , produced by Kianshar D company enable performing the titration by just pressing a botton and provide the results within a few minutes. This ability is achieved by using some special components such as :
special buret with a motorized piston which enables accurate dosing of the Karl Fischer reagents into the titration cell. The volume of this buret is usually 10ml and the motorized piston is so precise to can manage the minimum dosing of 0.0001 ml in each stept near the end point of the titration. In practice it results in really accurate end points.
3 way tap, which manage transferring of KF reagent from the container to the buret and from buret to the titration cell.
magnetic stirrer which mixes the solvent , sample and KF reagent by appropriate speed .
Titration cell including the sealed cap which has holes for titration tip , adding the sample and placing a small drying tube.
vacuum pump which is used for transferring the solvent into and the waste out of the titration cell. double platinum electrode as the main part of the bi-voltammetric indication system . During titration a constant current , 50 µA for example passes through the double platinum electrode. By when the sample contains water the KF reaction proceeds and it stops as soon as all the water content removed from the titration cell.The cell needs to be kept at high voltage in order to sustain the polarization current of the electrode at the required preset level. Once the titration is complete, free iodine will be found in the solution, which leads to a voltage drop and thus the polarization current level.
In modern Karl Fischer titrators such as KFT50 Titrika there is a control system which allows the fast reagent addition to the titration cell without any mistake in finding the end point. This is done by a special self adjustment of the control to the titration curve. By the other words the system enables the reagent addition in high speed at the first step of titration and then decreases the speed while the end point is getting closer.This kind of control allows the titration to be completed with high accuracy within a few minutes. --------------------------------------------------------------------------------------------------------------------------------------------------
* Water equivalent is the amount of water taken up by 1 ml of reagent
Sources
• chem.uiowa.edu/.../LUCIO%20GM%20KF-Titration%20March-2013.pdf
• dmsc2.dmsc.moph.go.th/.../Karl%20Fischer%20Titration.pdf
• www.labicom.cz/.../HYDRANAL-Seminar-2016_Praha_Brno.web_.pdf
• www.gpsil.co.uk/.../#
• http://kianshardanesh.com/article/106/Water%20in%20engine%20coolant